2
production plants, 1trading company

4
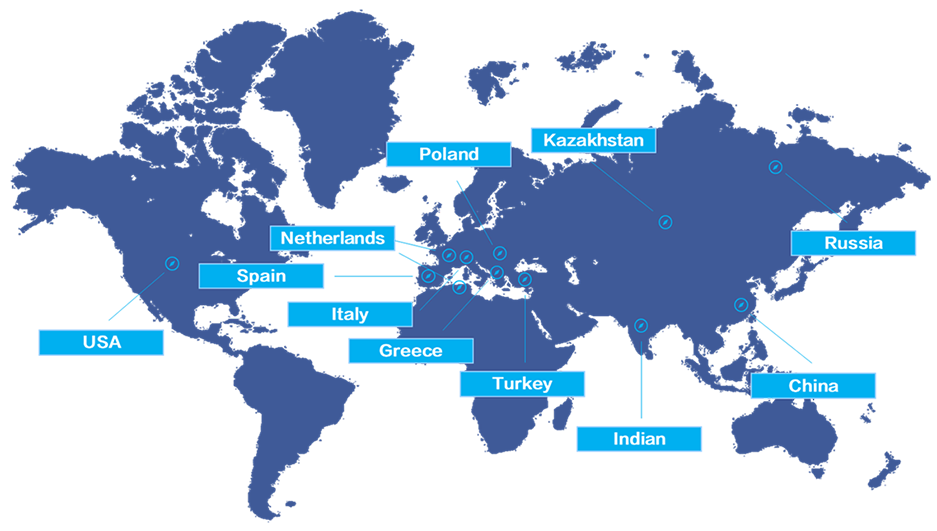
international business office in :
Russia, Poland, Italy, USA

5
US-DMF

$300M
Turnover

640
employees

1
CEP number
Kingland PharmCompany History
Kingland PharmOur Advantages

30 years working experience in Regulated Market

Local BD team for communication

Solving complex supply chain problems

Solid financial support

Solid relationship with suppliers

Professional team for registration and GMP compliance

Tailor to customers needs

Risk control on quality-supplying
KINGLAND PHARMOverseas Registration and GMP consultancy
01
Initial Vendor Evaluation 02
Project marketing analysis and expanding 03
Support on product technology on synthesis and analytical issue 04
Support on DMF(eCTD) to USFDA and EDQM for API registration 05
Doing mock authority inspection and gap evaluation 06
On-site training on quality system management 07
Support to prepare customer audit and CAPA response 08
Support to prepare authority inspection and CAPA responseKINGLAND PHARMTypical Products
| Product Name | ASMF | CEP | US DMF | GMP Certificate |
|---|---|---|---|---|
| Semaglutide | Available | Non-available | Available | WC available, |
| Alogliptin Benzoate | Available | Non-available | Non-available | WC available, |
| Amphotericin B | Available | Available | Available | WC available, "A"in CDE |
| Bicalutamide CEP grade | Available | Available | Non-available | WC under application, "I"in CDE |
| Biotin CEP grade | Available | Available | Available | WC available, "I"in CDE |
| Calcium Gluconate | Available | Non-available | Non-available | WC available, "A"in CDE |
| Carboplatin | Available | Non-available | Non-available | WC available, "I"in CDE |
| Cenobamate | Non-available | Non-available | Non-available | "I"in CDE |
| Ciclosporin | Available | Available | Available | WC available, "A"in CDE |
| Cisplatin | Available | Non-available | Non-available | WC Available |
| Cytisine | Available | Non-available | Available | WC available, Approved 19 countries in EU |
| Deflazacort | Available | Non-available | Non-available | WC available |
| Dexketoprofen | Available | Non-available | Non-available | WC under application |
| Dexmedetomidine | Available | Non-available | Non-available | WC available, "A"in CDE |
| Diquafosol Sodium | Available | Non-available | Non-available | WC available, "A"in CDE |
| DL-Serine | Non-available | Non-available | Non-available | Key intermediate under cGMP management |
| Dutasteride | On going | Non-available | Non-available | WC under application, "I"in CDE |
| Edaravone | Available | Non-available | Non-available | WC available, "A"in CDE |
| Empagliflozin | Available | Non-available | Non-available | WC available, "I"in CDE |
| Eribulin | Available | Non-available | Available | WC available, "I"in CDE |
KINGLAND PHARMMarketing